Introduction
Endocytosis refers to the active processes involved in bringing external substances into the cell using vesicles known as endosomes. There are a number of internalization pathways involved in order for cargo to pass through the cell membrane and into the cell, both for beneficial compounds and for harmful substances such as toxicants and viruses.
Endocytic pathways are therefore vital for cell health and drug delivery, meaning that assays for study of endocytosis and endosome formation are important to prevent/inhibit viral infections and to improve the efficiency of drug internalization.
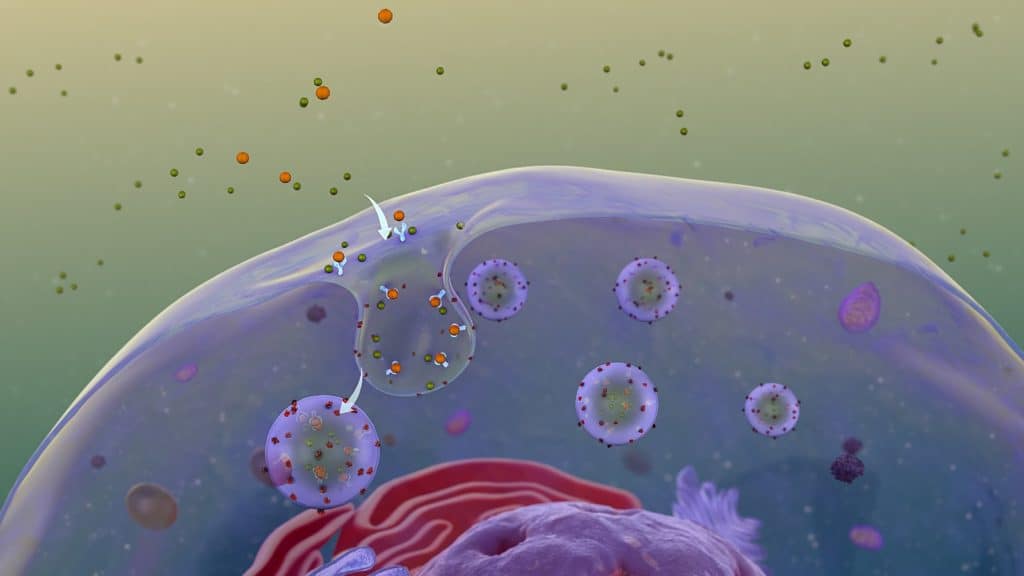
| Figure 1. Example of endocytosis. Particles outside the cell can be actively taken into the cell by the formation of endosomes (Image from WikiComms Receptor Mediated Endocytosis.jpg ) |
Endocytosis Pathways
The endocytic pathways used by a particular cell changes based on the cell type and the physical properties of the cargo (size, shape, charge, etc.). During endocytosis, cargo is taken into the cell by the local cell membrane forming into an early endosome, this is either recycled back towards the cell surface, or taken onwards to the late endosome, which ultimately fuses with the lysosome for breakdown. The initial internalization of cargo varies, study of endocytosis is an actively evolving field and at the time of writing there are five major types:
1. Clathrin-mediated endocytosis (CME)
CME (also called receptor-mediated endocytosis) is the best understood endocytic pathway, as it is the principal route for nutrients (such as iron and cholesterol) in all mammalian cells. CME uses clathrin-coated pits on the cell surface, which invaginate to form an intracellular clathrin-coated vesicle to take cargo into the cell.
2. Fast endophilin-mediated endocytosis (FEME)
FEME is a recently discovered pathway for specific transmembrane receptors that rapidly (<10 s) forms a vesicle when ligands bind to these receptors, using dynamin rather than clathrin.
3. Clathrin-independent carrier (CLIC) or glycosylphosphatidylinositol-anchored protein enriched early endocytic compartment (GEEC)
CLIC/GEEC is similar to FEME but occurs continuously without ligand binding, transports different cargo, and is independent of both dynamin and clathrin.
4. Phagocytosis
Critical for uptake of dead cells, pathogens and debris, certain phagocytes (macrophages, monocytes etc.) engulf/eat their target and form a phagosome in response to the binding of certain ligands.
5. Pinocytosis
While phagocytosis involves ‘eating’, pinocytosis involves ‘drinking’, and mostly transports extracellular fluid and small particles into the cell. Novel pathway is macropinocytosis, which creates cell-surface ‘ruffles’ that collapse back into the cell and form large fluid-filled macropinosomes.
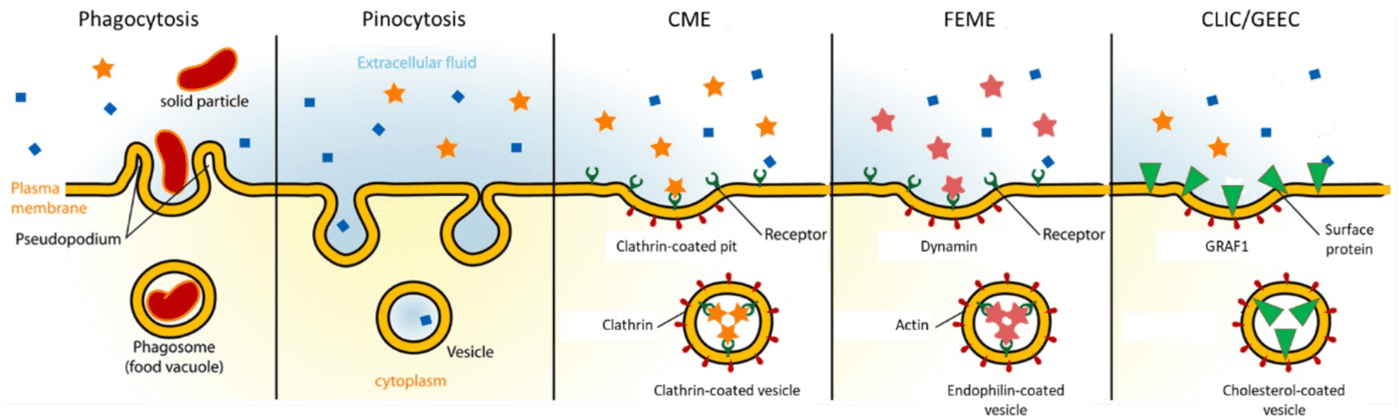
| Figure 2. Different endocytosis pathways and methods of endosome formation. Diagram shows extracellular area (blue, top of figure), the cell plasma membrane (yellow) and the intracellular area (orange, bottom of figure), including the ligands, proteins and receptors involved in the different pathways. |
Another pathway is caveolae, but there is limited evidence of cargo dependent on caveolae, this pathway may be being confused with FEME and CLIC/GEEC due to the poor specificity of endocytosis inhibitors and other research tools (Parton et al. 2018; Rennick et al. 2021). This further highlights the need for additional study and improved tools.
Endocytosis Assays
Endocytosis and associated pathways can be studied using a wide variety of assays, detailed in this section.
Membrane Observation
Endocytosis can be observed in situ using highly sensitive microscopy, typically total internal reflection fluorescence (TIRF). TIRF with live cells allows for direct visualization of endocytosis dynamics in real time, even isolating individual events. TIRF maintains a thin illumination field ~200 nm into the sample, effectively isolating the basal membrane of cells on coverslips. However, this works best with adherent cells (making suspension cells challenging to image), and only captures events in the membrane in contact with the coverslip. Examples and protocols can be found via Soohoo et al. (2014) or Zhang et al. (2018).
pH-sensitive probes
Endosomes are typically more acidic than the extracellular environment, (extracellular pH ~7.4, endosomes pH ~6.3, lysosome pH ~4.7) and this change in pH upon internalization can be monitored by labelling the cargo with pH-sensitive fluorescent probes, which will reveal endosomal formation and intracellular location with high temporal resolution and quantitative data (Sposini et al. 2020).
Endocytic inhibition
Chemicals and toxicants (such as statins) can be used to inhibit certain endocytosis pathways and observe the effects on the cell. However, these effects are not specific and may block multiple pathways or have unforeseen secondary effects on the cell. Many clathrin and dynamin inhibitors are available but should be characterized with your specific cell type first.
Antibody uptake
If your experiment uses a known cargo, antibodies specific to that cargo can be combined with fluorophores and used as highly selective tags. Cells are incubated with antibody-labeled cargo for a set time for uptake to occur, this can then be paused by refrigeration and remaining extracellular cargo remaining can be labelled with different markers, allowing for uptake to be quantified using techniques such as flow cytometry.
Fluorescent labelling
Fluorescent proteins and dyes are used, the former in the walls of an endosome and the latter as the cargo, both can track the formation, transport and breakdown of endosomes. Knowing the endocytic pathway, cell type, and target cargo is necessary as fluorescent labels can then be used to tag initializing ligands, the cargo itself, or any membrane surface proteins involved in transport.
Summary
Endocytosis is an essential process and can often be disrupted during disease, or manipulated by pathogens/toxicants (such as viruses) to gain entry into cells. Further study of endocytosis is vital for more effective drug delivery and potential future viral therapies.
References
Parton, R. G. (2018) Caveolae: structure, function, and relationship to disease. Annu. Rev. Cell Dev. Biol. 34, 111–136.
Rennick, J.J., Johnston, A.P.R. & Parton, R.G. (2021) Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276. https://doi.org/10.1038/s41565-021-00858-8
Soohoo, A. L., Bowersox, S. L., & Puthenveedu, M. A. (2014). Visualizing clathrin-mediated endocytosis of G protein-coupled receptors at single-event resolution via TIRF microscopy. Journal of visualized experiments : JoVE, (92), e51805. https://doi.org/10.3791/51805
Sposini, S., Rosendale, M., Claverie, L. et al. (2020) Imaging endocytic vesicle formation at high spatial and temporal resolutions with the pulsed-pH protocol. Nat Protoc 15, 3088–3104. https://doi.org/10.1038/s41596-020-0371-z
Zhang, Z., Heidary, D. K., & Richards, C. I. (2018). High resolution measurement of membrane receptor endocytosis. Journal of biological methods, 5(4), e105. https://doi.org/10.14440/jbm.2018.266