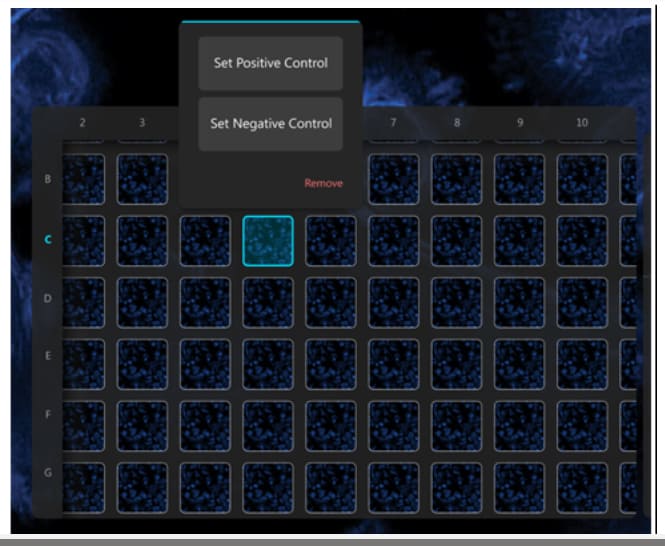
How do you ensure your high content analysis (HCA) data is of high quality?
Like all other lab techniques, quality HCA experiments (and subsequent data) depend on the use of the correct control samples. For many new users, this is an often overlooked but crucially important step of the process. Luckily, in a multiwell plate format, it is easy to add in the correct controls for every experiment.
There are two main classes of controls – experimental or treatment controls, and labeling controls. We will discuss both below.
Experimental controls
Experimental controls allow you to assess the effects of your test compounds with confidence. They can be broken down into three classes:
- Positive control: This sample is essential for determining the dynamic range and sensitivity of your assay. Positive control samples are fluorescently labeled samples that are treated with a compound with a known effect (Figure 1A). For example, nocodazole is known to cause inhibition of neurite formation, and is often used as a positive control in neurite outgrowth assays. The positive control allows serves as a reference when setting exposure settings. When your cells do not show a standard response to the positive control, it can indicate issues with the cells or experimental design.
- Vehicle-only control: Vehicle controls are fluorescently labelled cells that are treated with the substance in which your compounds of interest are dissolved (Figure 1B). The role of the vehicle control is to determine whether the changes observed are due to the compound of interest or the vehicle. Commons vehicle control substances include DMSO, ethanol and corn oil. In a well-designed assay, the vehicle control response will be like the negative control response.
- Negative control: A negative treatment control is a cell that is fluorescently labelled, but not treated with any compounds (Figure 1C). The role of the negative control is to serve as a baseline reference for the state of the cells, and to demonstrate that the effects observed are due to the treatment compounds.
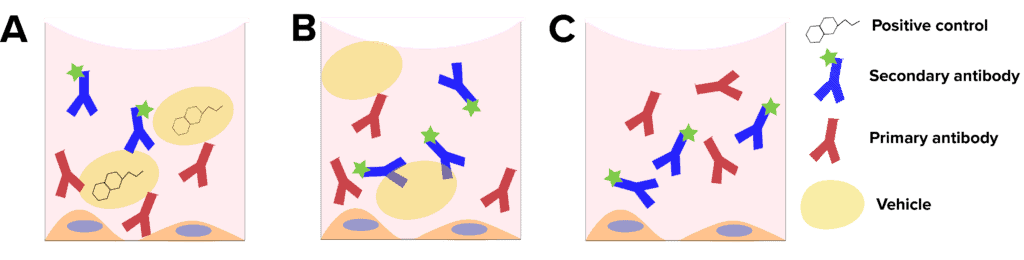 Figure 1: Experimental controls. A, Positive controls contain a compound known to cause the desired response. B, vehicle controls contain only the solvent of the compounds, with no target compound. C, negative controls do not contain vehicle or test compounds.
Figure 1: Experimental controls. A, Positive controls contain a compound known to cause the desired response. B, vehicle controls contain only the solvent of the compounds, with no target compound. C, negative controls do not contain vehicle or test compounds.
Labeling controls
Because HCA is based on automated fluorescence microscopy, it is essential to include more traditional microscopy controls in each experiment. Labelling controls help to ensure that the fluorescence signal detected is the result of the experimental conditions, not underlying, native fluorescence or non-specific antibody binding.
There are two types of essential labelling controls for high content analysis:
- Autofluorescence control: It is essential to include an unlabeled control with each experiment. To generate this sample, you do no staining (Figure 2A). This sample is imaged with the same conditions as all other samples, and if fluorescence is detected, that is considered a background signal or autofluorescence. Certain cell types are prone to showing autofluorescence, and some types of cell culture media have low levels of fluorescence as well. It may be necessary to run a autofluorescence control for each compound you are assessing.
- Secondary-only control: When utilizing antibody-based staining, it is critical to include secondary only controls in all experiments, for all secondary antibodies you are using. For this control sample, you do not incubate your sample with the primary antibody, instead only using the secondary antibody (Figure 2B). This control lets you determine that there is no non-specific binding of that antibody to the cells (or plate) and give you confidence that the fluorescent signal you are detecting is a result of the assay. If you do detect non-specific staining, it is critical to reexamine your blocking and wash steps used in your staining protocol, and it may be necessary to adjust percentages.
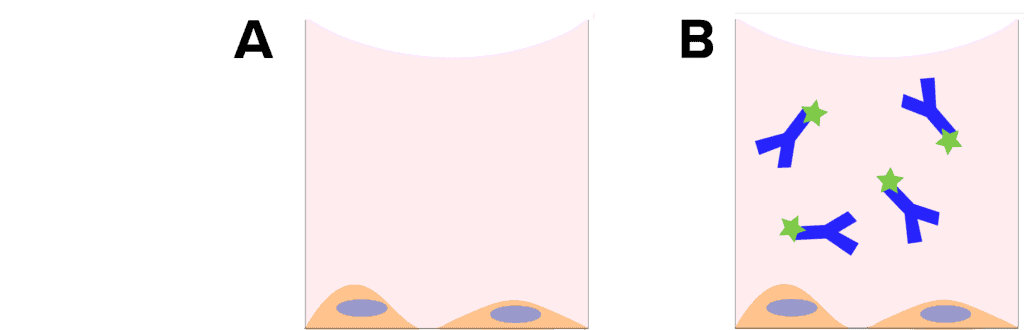 Figure 2: Labelling controls. A, autofluorescence controls containing no stain or antibodies. B. Secondary only control, to detect nonspecific binding of the secondary antibody.
Figure 2: Labelling controls. A, autofluorescence controls containing no stain or antibodies. B. Secondary only control, to detect nonspecific binding of the secondary antibody.
Once the controls are set up and you are ready to acquire data, you can import this information into the Araceli Voyager software. Setting this in the software means that assay robustness scores can be calculated while images are acquired, leaving no question as to whether you have acquired quality data.